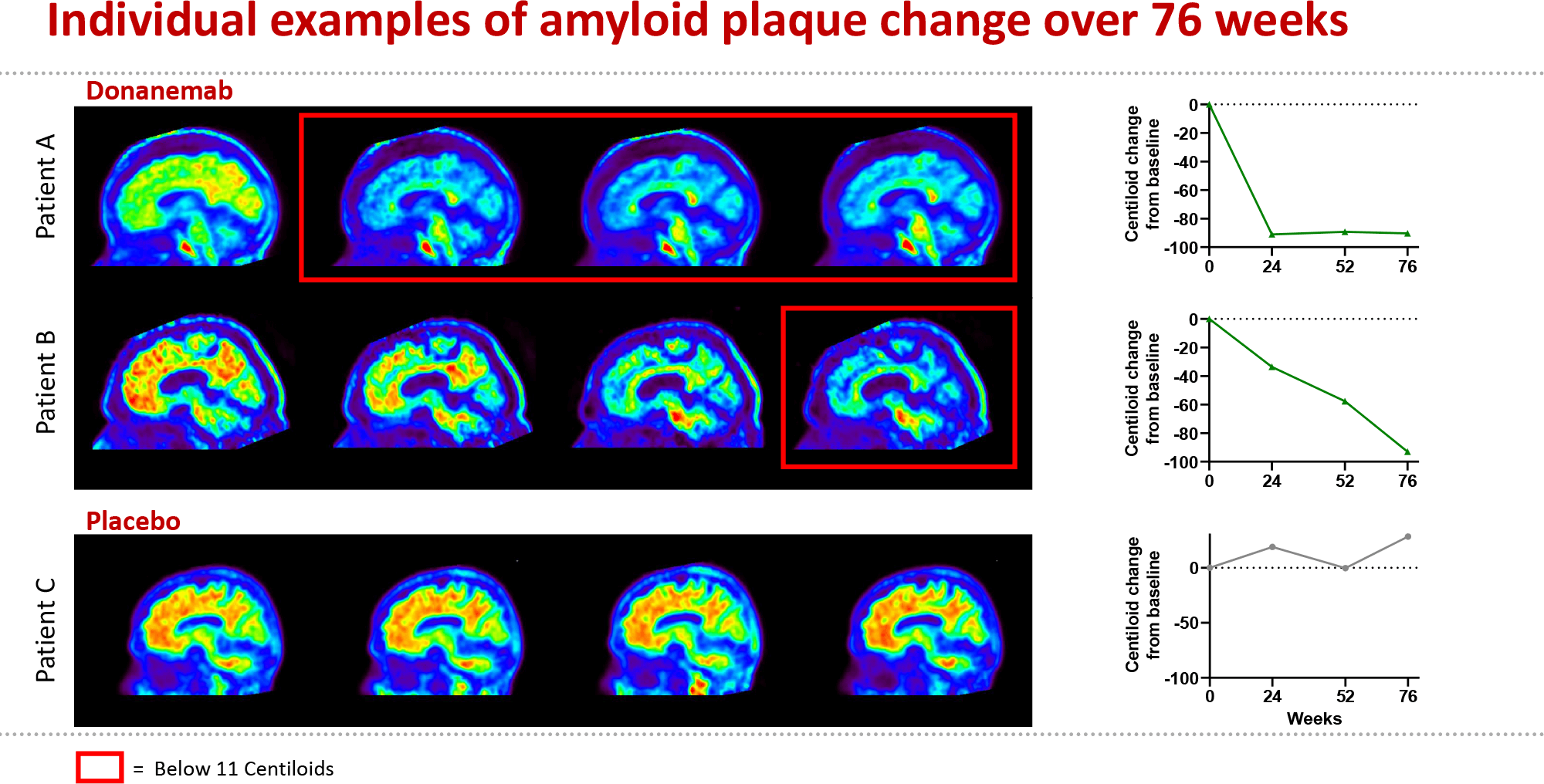
Following the results of the donanemab phase 3 data, Dr Richard Oakley, Associate Director of Research at Alzheimer’s Society, said: “After 20 years with no new Alzheimer's drugs, we now have two potential new drugs in just twelve months – and for the first time, drugs that seem to slow the progression of disease. This could be the beginning of the end of Alzheimer’s disease.
“Based on today’s early results, donanemab appears to slow the progression of Alzheimer’s symptoms by 36 % (as compared with 27% of last year’s breakthrough drug lecanemab). Promisingly, the trial also demonstrated a 40% slowing in decline of everyday activities such as driving, doing hobbies and managing finances.
“While we’ve seen lecanemab could slow progression by over seven months, we’ll need to see the full results to know if donanemab could do the same or even better. We’re so proud that Alzheimer’s Society funded researchers discovered the role of amyloid in Alzheimer’s disease over 30 years ago which made today’s breakthrough possible.
“We need decisions as quickly as possible from the regulators MHRA and NICE. But that’s not the end of the story - we can’t end up in a situation where there are new drugs being approved but people can’t get access to them early in their dementia journey when they work best – we need more accurate, earlier dementia diagnosis in the NHS.”